Xanthan gum is a polysaccharide used as a common food additive but with many industrial uses. It is an effective thickening agent, emulsifier, and stabilizer that prevents ingredients from separating. It can be produced from simple sugars using a fermentation process and derives its name from the species of bacteria used, Xanthomonas campestris.
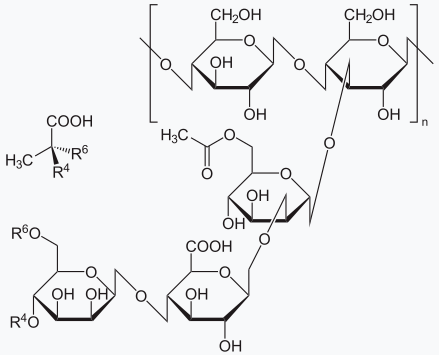
Molecular Structure
Xanthan gum was discovered at the United States Department of Agriculture, and brought into commercial production by CP Kelco under the trade name Kelzan in the early 1960s. It was approved for use in foods in 1968 and is accepted as a safe food additive in the US, Canada, European countries, and many other countries.
Properties
Xanthan gum soluble in both cold and hot water and is generally not affected by changes in pH value. Xanthan gum will dissolve in most acids or bases. Xanthan gum binds water as all hydrocolloids.
The viscosity of xanthan gum is stable at low pH values and at high temperatures for a long period of time and is not affected by the addition of large amounts of salt. Thanks to its water binding capacity, xanthan gum solutions exhibit good freeze/thaw stability.
By itself, xanthan drastically increases the viscosity (thickness) of any liquid it is added to in very low concentrations. In high concentrations, it will form a mucusy paste that looks like a gel but is not technically a gel.
Xanthan gum has a synergistic effect in combination with locust bean gum and konjac (gel formation) as well as with guar gum (higher viscosity). Thanks to the unique rheological and synergistic properties of its aqueous solutions, xanthan gum is used in many applications as a suspending agent and emulsion stabilizer, a foam enhancer or an improver of dough volume.The viscoelasticity between xanthan and locust bean gums is due to the cross-linking between smooth region of locust bean gum and disordered segment of xanthan.
Uses
Xanthan gum is one of the most successful hydrocolloids due to its unique functionality, particularly in difficult environments like acid, high salt and high shear stress.
Xanthan gum has the E Number E 415.
One excellent use of xanthan gum is for sauces since it prevents syneresis.
By increasing the viscosity of liquids, it helps to prevent syneresis in gels, keep ice crystals from forming in frozen goods, and help stabilize emulsions and foams. Xanthan is a popular ingredient in gluten-free foods because it can impart some of the texture that gluten gives to baked goods.
Xanthan gum is described by many chefs as the most versatile of the hydrocolloids. It is very easy to use use in either cold or hot applications and only a very small amount is needed. Unlike most starches it does not require heat to be hydrated which make it ideal for all sauces.. Xanthan gum is also very stable over a wide range of temperatures, in various acid, alkaline and salty mixtures.
In molecular gastronomy it is widely used for spherification, to suspend solids in liquids thanks to its elasticity, for thickening sauces without altering the mouth feel, for gas retention in liquids, and to help stabilize emulsions and foams.
