
Pectin is a polysaccharide consisting of a long chain of pectic acid and pectinic acid molecules. It is produced by extraction from citrus peels and the remains of apples (apple pomace) after apple juice processing. In fruit, pectin is the material that joins the plant cells together. When enzymes break down the pectin in the fruit, the fruit gets soft and mushy.
Citrus fruits

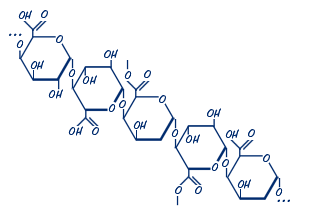
Molecular Structure
Properties
Isolated pectin has a molecular weight of typically 60,000 to 130,000 g/mol, varying with origin and extraction conditions.
In nature, around 80 per cent of carboxyl groups of galacturonic acid are esterified with methanol. This proportion is decreased to a varying degree during pectin extraction. Pectins are classified as high- versus low-methoxy pectins (HM-pectins versus LM-pectins), with more or less than half of all the galacturonic acid esterified. The ratio of esterified to non-esterified galacturonic acid determines the behaviour of pectin in food applications – HM-pectins can form a gel under acidic conditions in the presence of high sugar concentrations, while LM-pectins form gels by interaction with divalent cations, particularly Ca2+, according to the idealized ‘egg box’ model, in which ionic bridges are formed between calcium ions and the ionised carboxyl groups of the galacturonic acid.
In high-methoxy pectins at soluble solids content above 60% and a pH value between 2.8 and 3.6, hydrogen bonds and hydrophobic interactions bind the individual pectin chains together. These bonds form as water is bound by sugar and forces pectin strands to stick together. These form a three-dimensional molecular network that creates the macromolecular gel. The gelling mechanism is called a low-water-activity gel or sugar-acid-pectin gel.
While low-methoxy pectins need calcium to form a gel, they can do so at lower soluble solids and higher pH than high-methoxy pectins. Normally low-methoxy pectins form gels with a range of pH from 2.6 to 7.0 and with a soluble solids content between 10 and 70%.
The non-esterified galacturonic acid units can be either free acids (carboxyl groups) or salts with sodium, potassium, or calcium. The salts of partially esterified pectins are called pectinates; if the degree of esterification is below 5 per cent, the salts are called pectates, the insoluble acid form, pectic acid.
Some plants, such as sugar beet, potatoes and pears, contain pectins with acetylated galacturonic acid in addition to methyl esters. Acetylation prevents gel formation but increases the stabilising and emulsifying effects of pectin.
Amidated pectin is a modified form of pectin. Here, some of the galacturonic acid is converted with ammonia to carboxylic acid amide. These pectins are more tolerant of varying calcium concentrations that occur in use.
To prepare a pectin-gel, the ingredients are heated, dissolving the pectin. Upon cooling below gelling temperature, a gel starts to form. If gel formation is too fast or too strong, syneresis or a granular texture is the result, while weak gelling leads to excessively soft gels (called pre-gelation).
Amidated pectins behave like low-ester pectins but need less calcium and are more tolerant of excess calcium. Also, gels from amidated pectin are thermoreversible; they can be heated and, after cooling, solidify again, whereas conventional pectin gels are thixotropic (they become liquid when stirred and gel again afterwards).
High-ester pectins set at higher temperatures than low-ester pectins. However, gelling reactions with calcium increase as the degree of esterification falls. Similarly, lower pH values or higher soluble solids (normally sugars) increase gelling speeds. Suitable pectins can therefore be selected for jams and jellies or for higher-sugar confectionery jellies.
Uses
The main use for pectin is as a gelling agent, thickening agent and stabiliser in food. The classical application is in jams, jellies and marmalades, where it provides the gel. Pectin also reduces syneresis in jams and marmalades and increases the gel strength of low-calorie jams. For household use, pectin is an ingredient in gelling sugar (also known as “jam sugar”), where it is diluted to the right concentration with sugar and some citric acid to adjust pH. In some countries, pectin is also available as a solution or an extract or as a blended powder for home jam-making.
For conventional jams and marmalades that contain above 60% sugar and soluble fruit solids, high-ester pectins are used. With low-ester pectins and amidated pectins, less sugar is needed, so diet or low-calorie products can be made.
Pectin is used in confectionery jellies to give a good gel structure, a clean bite and to confer a good flavour release. Pectin can also be used to stabilise acidic protein drinks, such as drinking yogurt, to improve the mouth-feel and the pulp stability in juice-based drinks and as a fat substitute in baked goods. Typical levels of pectin used as a food additive are between 0.4 and 1.0% – this is about the same amount of pectin as in fresh fruit.
In medicine, pectin increases the viscosity and volume of stool so that it is used against constipation and diarrhea. Until 2002, it was one of the main ingredients used in Kaopectate – a medication to combat diarrhea – along with kaolinite. It has been used in gentle heavy metal removal from biological systems. Pectin is also used in throat lozenges as a demulcent. Pectin is also used in wound healing preparations and speciality medical adhesives, such as colostomy devices.
In cosmetic products, pectin acts as a stabiliser.
In cigars, pectin is considered an excellent substitute for vegetable glue, and many cigar smokers and collectors use pectin for repairing damaged tobacco leaves on their cigars.
